Scientists from Korea in collaboration with scientist at washington University, Missouri recently evaluated the catalytic properties of Au-based nanostructures (including nanocages, nanoboxes, and solid nanoparticles) using a model reaction based on the reduction of p-nitrophenol by NaBH4
.
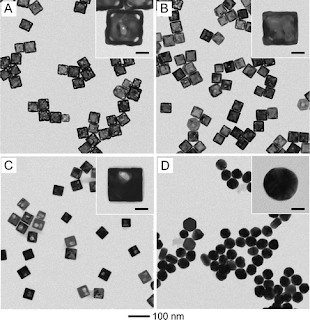
It is well-known that the catalytic activity of a nanoparticle is strongly dependent on its size. Typically, a smaller nanoparticle tends to show a higher catalytic activity as it has a much greater surface-to-volume ratio.However, smaller nanoparticles may not be better candidates for catalyzing all types of reactions. A good example for explaining this exception can be found in a redox reaction. As the catalytic particles become increasingly smaller, the oxidation and reduction half reactions might need to occur on different particles due to the reduction in surface area. In this case, a good “electrical” connection between the particles will play an important role as the electrons have to be transported from the site of oxidation to the site of reduction. The redox reaction will be unable to proceed if the catalytic particles are separated from each other by an insulating medium.It has been argued that this kind of problem can be solved by switching from solid nanoparticles to nanocages or nanoboxes with hollow interiors and ultrathin walls. For a Au-based nanocage of 50 nm in edge length and 5 nm in wall thickness, it should be able to provide a sufficiently large surface area (at least, equivalent to a 50 nm solid particle) to accommodate both the oxidation and reduction half reactions while the ultrathin wall is still able to provide a high activity equivalent to a 5 nm solid particle due to a good electrical connection across the entire surface of the wall. For a model reaction based on the reduction of p-nitrophenol by sodium borohydride (NaBH4), The experimental data reported indicate that both wall thickness and porosity of the Au-based hollow nanostructures play an important role in enhancing the catalytic activity. The kinetic data in their report (published in Nano letters) indicate that the Au-based nanocages are catalytically more active than both the nanoboxes and nanoparticles probably due to their extremely thin but electrically continuous walls, the high content of Au, and the accessibility of both inner and outer surfaces through the pores in the walls.
In summary, the good intrinsic electrical connection across the entire surface of a Au nanocage makes it a much better catalyst than small Au solid nanoparticles for a redox reaction. In addition, a typical compensation effect was observed in this catalytic system, which can be explained by the assumption of kinetic regime switching. Given the high abundance of Au element relative to other noble metals like Pt and Pd as well as the easiness in controlling the porosity and morphology, the Au-based nanocages might be able to find widespread use as catalysts in a number of industrial applications.
1 comment:
I really appreciate your post and you explain each and every point very well.Thanks for sharing this information.And I’ll love to read your next post too.
Regards
iso 9000
Post a Comment