Saturday, November 28, 2009
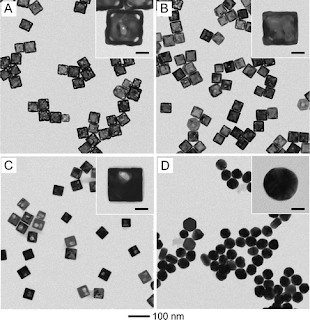
Gold based Nanocages and Nanoboxes: Effective Catalysts for Redox Reaction

India's ambitious 'Solar Mission'

India's prime minister Manmohan Singh has approved a US$19 billion plan to make the country a global leader in solar energy over the next three decades. The ambitious project would see a massive expansion in installed solar capacity, and aims to reduce the price of electricity generated from solar energy to match that from fossil fuels by 2030.
The 'solar mission' was first mooted as part of India's national action plan on climate change, announced in June 2008. According to a draft mission document whose targets were approved on 3 August, installed solar capacity would be hiked from its current 5 MW to 20 GW by 2020, 100 GW by 2030 and 200 GW by 2050 — more than the current 150 GW power generation capacity of all India's coal, gas and nuclear plants.
Officials say the plan shows that the country is serious about its intention to stem global warming, ahead of the UN climate change conference in Copenhagen in December.
A detailed road map has been drawn up to 2020. By then, according to the mission document, solar lighting will be available for 20 million households and 42 million tonnes of CO2 emissions will be saved annually by the switch to solar energy. The government plans to create a solar fund with initial investment of $1.1 billion and build it up by taxing fossil fuels and the power generated from them — 0.1 cents for every kWh produced. By 2030, it hopes to reduce the cost of electricity from photovoltaic cells to around 10 cents per kWh, matching the price of electricity derived from conventional fuels.
The plan will be pushed forward by a mixture of other policy and regulatory measures. Those include making it mandatory for existing thermal power plants to generate at least 5% of their capacity from solar power, and for government buildings to install photovoltaic panels on rooftops. Producers connected to the grid will be able to sell their excess solar electricity to utilities; solar-power projects get a 10-year tax holiday; and other 'carrots' for the industry include the duty-free import of raw materials and priority bank loans.
An autonomous solar-energy authority will be created to execute the mission, but the existing solar-energy centre near New Delhi will be upgraded into an 'apex research institute' to coordinate solar-research centres across the country and promote foreign collaboration. The mission document recommends introducing solar-energy courses to the Indian Institutes of Technology, and creating a fellowship programme to train 100 Indian scientists a year in world-class institution
Tuesday, November 24, 2009
Developing Complex Patterns on Surface
 upling. Fluorophores were attached to the deprotected regions, providing visual evidence of surface patterning using fluorescence microscopy. Their approach is universal to bind moieties containing free amine groups at defined regions across a surface, allowing for the development of films with complex chemical and physicochemical properties.
upling. Fluorophores were attached to the deprotected regions, providing visual evidence of surface patterning using fluorescence microscopy. Their approach is universal to bind moieties containing free amine groups at defined regions across a surface, allowing for the development of films with complex chemical and physicochemical properties.Monday, November 23, 2009
Biomedical Imaging of Cells using Quantum Dots
These quantum dots last longer than most of the organic dyes and fluorescent proteins that we previously used to illuminate the interiors of cells. They also have the advantage of monitoring changes in cellular processes while most high-resolution techniques like electron microscopy only provide images of cellular processes frozen at one moment. Using quantum dots, cellular processes involving the dynamic motions of proteins can be elucidated.
In the recent study, the research team focused primarily on characterizing quantum dot properties, contrasting them with other imaging techniques. In one example, they employed quantum dots designed to target a specific type of human red blood cell protein that forms part of a network structure in the cell's inner membrane. When these proteins cluster together in a healthy cell, the network provides mechanical flexibility to the cell so it can squeeze through narrow capillaries and other tight spaces. But when the cell gets infected with the malaria parasite, the structure of the network protein changes.
Since the clustering mechanism is not well understood, it was examined with the dots. Researchers believed that if they could develop a technique to visualize the clustering, they could learn something about the progress of a malaria infection, which has several distinct developmental stage.

fig : Human red blood cells, in which membrane proteins are targeted and labeled with quantum dots, reveal the clustering behavior of the proteins. The number of purple features, which indicate the nuclei of malaria parasites, increases as malaria development progresses. The NIST logo at bottom was made by a photo lithography technique on a thin film of quantum dots, taking advantage of the property that clustered dots exhibit increased photoluminescence. (White bars: 1 micrometer; red: 10 micrometer)
The team's efforts revealed that as the membrane proteins bunch up, the quantum dots attached to them are induced to cluster themselves and glow more brightly, permitting scientists to watch as the clustering of proteins progresses. More broadly, the team found that when quantum dots attach themselves to other nanomaterials, the dots' optical properties change in unique ways in each case. They also found evidence that quantum dot optical properties are altered as the nanoscale environment changes, offering greater possibility of using quantum dots to sense the local biochemical environment inside cells. about the progress of a malaria infection, which has several distinct developmental stages.
Saturday, November 21, 2009
Nanostructured Integrated Circuits detect Type and Severity of Cancer
.gif)
Friday, November 20, 2009
Direct Imaging in Real Space and Time with 4D Electron Microscopy

Thursday, November 19, 2009
Ultraflat Graphene

Wednesday, November 18, 2009
One-Dimensional Arrangement of Gold Nanoparticles with Tunable Interparticle Distance

Tuesday, November 17, 2009
Newly Designed highly Fluorescent and Photostable Nanoparticles

Monday, November 16, 2009
Carbon Nanotubes Dramatically Affecting Plant Growth !
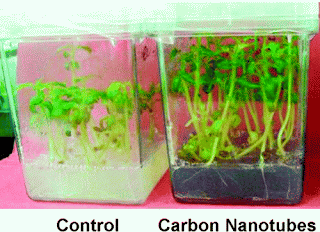 For the first time, Scientist at University of Arkansas have reported the effect of penetration of plant seed coats by carbon nanotubes. Their research demonstrate that the exposure of carbon nanotubes to seeds of valuable crops, such as tomatoes, can increase the germination percentage and support and enhance the growth of seedlings.
For the first time, Scientist at University of Arkansas have reported the effect of penetration of plant seed coats by carbon nanotubes. Their research demonstrate that the exposure of carbon nanotubes to seeds of valuable crops, such as tomatoes, can increase the germination percentage and support and enhance the growth of seedlings.The germination was found to be dramatically higher for seeds that germinated on medium containing CNTs (10−40 μg/mL) compared to control. Analytical methods indicated that the CNTs are able to penetrate the thick seed coat and support water uptake inside seeds, a process which can affect seed germination and growth of tomato seedlings.The activated process of water uptake could be responsible for the significantly faster germination rates and higher biomass production for the plants that were exposed to carbon nanotubes.
Researchers believe that furthering these findings could result in significant developments of improved plants for the area of energy, by taking advantage of the enhancement in the biomass of the plants when they are exposed to nanosized materials and fertilizers.
An observed positive effect of CNTs on the seed germination could have significant economic importance for agriculture, horticulture, and the energy sector, such as for production of biofuels.
paper can be located at ACS Nano, 2009, 3 (10), pp 3221–3227
Sunday, November 15, 2009
Enhanced Hydrogen Adsorptivity of Single-Wall Carbon Nanotube Bundles

 .
.In summary, these new results indicate a simple and promising tuning route for SWCNT bundle structures, allowing the utilization of interstitial nanopore spaces for various fields, such as electrochemical, adsorption, sensor, and separation technologies.
paper can be found at Nano Lett., 2009, 9 (11), pp 3694–3698
Saturday, November 14, 2009
Lead-Sulfide (PbS) Nanowire Pine Trees!

In contrast to previous PbS nanowire growth via CVD reactions with or without intentional metal catalysts, this tree morphology is formed by a combination of screw dislocation-driven nanowire growth that produced long and twisted “trunk” nanowires and a simultaneous self-catalytic vapor-liquid-solid (VLS) mechanism that resulted in epitaxial “branch” nanowires. Lead particles generated in situ are suggested to be a self-catalyst to enable VLS growth of the branches that grow epitaxially off the trunk.
The optimum conditions for synthesizing PbS nanowire pine trees have been investigated in this research with detailed studies of morphology changes under various hydrogen, temperature, pressure, and substrate conditions. The successful growth of nanowires driven by screw dislocations requires two basic ingredients: the creation (seeding) of dislocations and a suitably low supersaturation condition for promoting dislocation-driven growth over layer-by-layer growth and other growth modes.
The ability to control the formation of hierarchical nanostructures with increasing structural complexity, as seen in these pine tree nanowires, can potentially empower increasing functionalities and enhance applications such as solar energy conversion and 3-D nanoelectronics.
This paper can be found at J. Am. Chem. Soc., 2009, 131 (45), pp 16461–16471
Friday, November 13, 2009
True Nature of Gold's Bonding

Thursday, November 12, 2009
Environment-sensitive stabilisation of silver nanoparticles in aqueous solutions

Scientists from Netherlands have recently reported on the formation of silver containing composite nanoparticles (CNPs) consisting of silver nanoparticles (Ag-NPs), poly(N-methyl-2-vinyl pyridinium iodide)-block-poly(ethylene oxide), P2MVP38-b-PEO211 and poly(acrylic acid)-block-poly(isopropyl acrylamide), PAA55-b-PNIPAAm88. Both the Ag-NPs and the CNPs from spontaneously upon mixing of the double hydrophilic block copolymers in the presence of silver ions; that is, without the addition of a reducing agent such as NaBH4. Paper demonstrate the possibilities to achieve control over the size of the Ag-NPs, the size and shape of the CNPs, and the location of the Ag-NPs within the CNPs. Ag-NPs were found to colocalise with the polyelectrolyte blocks within the CNPs. Temperature could be used to trigger a structural transition from a core–shell structure at T = 25 °C to a core–shell–corona structure at T = 60 °C , translocating the Ag-NPs from the micellar core in the former into the micellar shell in the latter. The most uniform and well-defined CNPs were obtained by premixing Ag+ and P2MVP38-b-PEO211 at room temperature prior to addition of this solution to a solution of PAA55-b-PNIPAAm88 at T = 25 °C (sample H). It has been shown that the colloidal stability of the CNPs is dependent on the ionic strength of the solution: Ag-NP release from the CNPs can easily be triggered by addition of a simple salt, such as NaNO3.
This work summarizes that complex coacervate core micelles can be regarded as a promising candidate for polymer-assisted synthesis and stabilisation of silver nanoparticles. Future work might be directed towards the potential application of such CNPs as environment-sensitive silver quantum dots and as antimicrobial agents in antifouling surface coatings that can be prepared upon exposure of hydrophilic surfaces to a solution of Ag-NP containing CNPs.
paper can be found at Journal of colloid and Interface Science, Vol 339, 2, pp 317-324
Wednesday, November 11, 2009
Tetrahedral Gold nanocrystal

Scientist at Hongkong have recently grown Elongated tetrahexahedral Au nanocrystals in high yields using a seed-mediated growth method.
Tuesday, November 10, 2009
Carbon nanotube sponge!

Scientists from China have invented a carbon-based sponge that can soak up organic pollutants, such as oils and solvents, from the surface of water. No water is absorbed and the sponge can then be wrung out and reused, like an ordinary household sponge. Absorbing up to 180 times its own weight in organic matter, the sponge is light and tough and has the potential to dramatically enhance oil spill cleanup.
Monday, November 9, 2009
Magnetic charge behaving as electric charge for the first time
 The research at London Centre for Nanotechnology find prove for the existence of atom-sized magnetic charges called ‘magnetic monopoles’. These monopoles behave and interact just like more familiar electric charges.Research also demonstrates a perfect symmetry between electricity and magnetism – a phenomenon dubbed ‘magnetricity'.
The research at London Centre for Nanotechnology find prove for the existence of atom-sized magnetic charges called ‘magnetic monopoles’. These monopoles behave and interact just like more familiar electric charges.Research also demonstrates a perfect symmetry between electricity and magnetism – a phenomenon dubbed ‘magnetricity'.In order to prove experimentally the existence of magnetic current for the first time, the research team mapped Onsager's 1934 theory of the movement of ions in water onto magnetic currents in a material called spin ice. They then tested the theory by applying a magnetic field to a spin ice sample at a very low temperature and observing the process using muon relaxation at ISIS, a technique which acts as a super microscope allowing researchers to understand the world around us at the atomic level.
The experiment allowed the team to detect magnetic charges in the spin ice (Dy2Ti2O7), to measure their currents, and to determine the elementary unit of the magnetic charge in the material. The monopoles they observed arise as disturbances of the magnetic state of the spin ice, and can exist only inside the material.
This research, reported in Nature, was led by Steven Bramwell of the London Centre for Nanotechnology in the UK. Bramwell was a member of a team, led by Tom Fennell of the Laue-Langevin Institute in Grenoble, that reported neutron results in September.